The genus Aitchisoniella contains a single species, A. himalayensis, which was described by Pakistani botanist Professor Shiv Ram Kashyap from plants that he collected in Mussoorie, Uttarakhand, India (1914). Subsequently (1929) he also found the species in Shimla and Kullu in Himachal Pradesh. Although reports of new locations followed (Kanwal, 1977, Pant et al., 1992, Bischler et al., 1994), near Nainital in Uttarakhand, the species was only known from the north-west Himalayas of India until 2010, when its range was extended by RBGE bryologist Dr David Long, on the ‘Kunming/Edinburgh Expedition to Sichuan’. David found Aitchisoniella at two localities in China, in Litang and Daocheng counties of south-west Sichuan Province.

Fig. 1. Athalamia pinguis in Sichuan, showing stalked sexual branches (carpocephala), photographed by David Long (Long # 40305)
Aitchisoniella looks quite different to other complex thalloid liverworts: it doesn’t have the stalked sexual branches (carpocephala) that are present in most complex thalloid species like Marchantia, or Athalamia (as can be seen in the photograph, Fig. 1). Instead, the female sex organs (archegonia), and therefore the sporophytes, grow on the lower (ventral) side of a short receptacle (as can be seen in the photograph, Fig. 2). The receptacle is part of the main thallus, with air chambers and air pores, and a groove on the underside of the thallus from which characteristicly complex-thalloid pegged rhizoids grow.

Fig. 2. Aitchisoniella himalayensis in Sichuan, showing terminal sporophyte-bearing receptacles, photographed by David Long (Long # 39886)
Originally, Aitchisoniella was thought to belong to the Exormothecaceae family of complex thalloid liverworts, along with Exormotheca and Stephensoniella. Earlier this year, we transferred all the plants in Exormothecaeae into another family, Corsiniaceae, where they joined Corsinia and Cronisia (Long et al. 2016a).
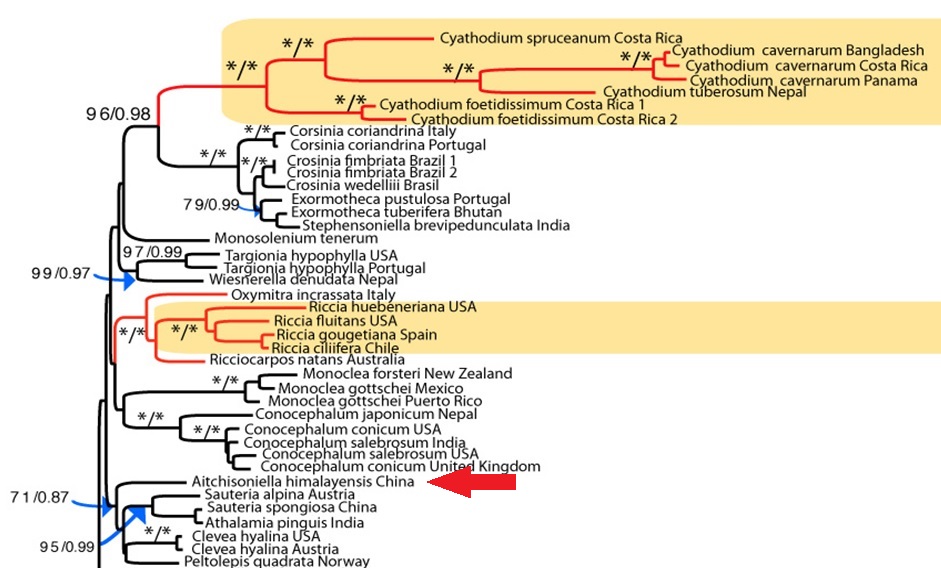
Fig. 3. The phylogenetic position of Aitchisoniella, from the complex thalloid phylogeny reconstructed by Villarreal et al. (2015)
However, having fresh material of the plant meant that we were able to extract DNA from it, and add it into our molecular phylogeny for the group (Villarreal et al. 2015). The results were unexpected, with Aitchisoniella grouping with species from a different complex thalloid family, Cleveaceae (Fig. 3). The growth forms are quite different, as all the species in Cleveaceae have got stalked carpocephala. However, once we started to think more about the evolution of these plants, and look more objectively at differences and similarities, one thing struck us: the spores of Aitchisoniella (see Fig. 4) look more like the spores of plants in Cleveaceae (e.g. Athalamia, Fig. 5) than they do like spores of plants in Exormothecaceae (e.g. Fig. 6).

Fig. 4. Spores of Aitchisoniella himalayensis adapted from Long et al. 2016: (A) Distal view; (B) proximal view; (C) lateral view; (D) detail of distal view. (A & C) from Long 39886. (B & D) from Long 40020. Scale bars: (A–C) = 5 μm, (D) = 2 μm.
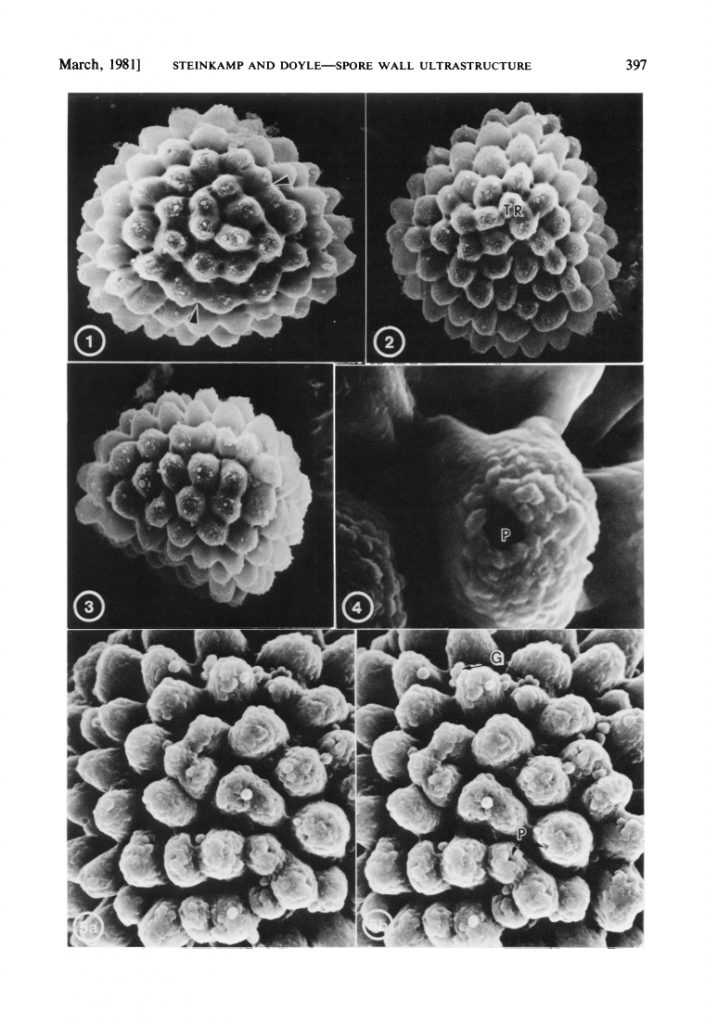
Fig. 5. Spores of Athalamia hyalina (Cleveaceae) adapted from Steinkamp & Doyle (1981): 1. distal view (x 1,100); 2. proximal view (x 1,100); 3. equatorial view (x 1,100); 4. close-up of pore (x 6,000); 5. distal face (x 3,000).
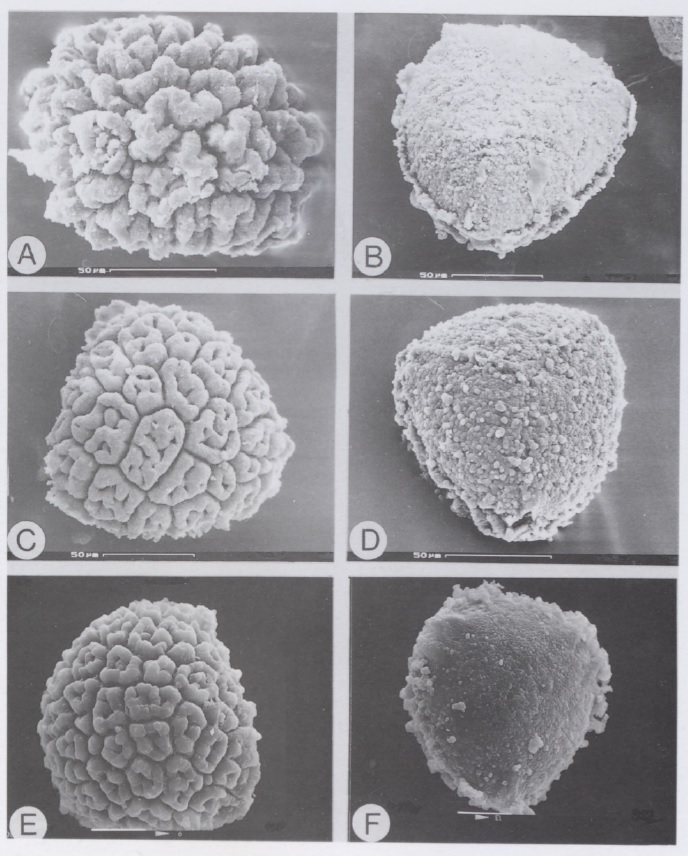
Fig. 6. Spores of Exormotheca adapted from Bornefeld et al. (1996): E. bulbigena – A, distal view, B, proximal view. E. holstii – C, distal view, D, proximal view, E, distal view, F, proximal view.
Characters like spore shape used to be considered to be quite neutral in bryophyte evolution, features that were not really acted on by natural selection. Following this theory, spore characters were thought to be indicative of true and ancient relationships, changing very little across huge amounts of time. We have since moved away from this view, with, for example, small changes in the shape and size of spores known to have drastic effects on their aerodynamics. However, within the complex thalloids, it does seem that characters like the presence or absence of carpocephalum branches are quite variable within families, while spore morphology can be indicative of deeper relationships.
As a result of this work, based on both molecular and morphological evidence, we have transferred the genus Aitchisoniella to the family Cleveaceae (Long et al. 2016b), where it joins the four genera accepted by Rubasinghe et al. (2011): Athalamia, Clevea, Peltolepis and Sauteria.
REFERENCES
Bischler, H., Boisselier-Dubayle, M.C. & Pant, G. 1994. On Aitchisoniella Kash. (Marchantiales). Cryptogamie. Bryologie-Lichénologie, 15: 103–10.
Borenfeld, T., O.H. Volk & R. Wolf. 1996. Exormotheca bulbigena sp. nov. (Hepaticae, Marchantiales) and its relation to E. holstii in southern Africa. Bothalia 26,2: 159–165.
Kanwal, H.S. 1977. Marchantiales of district Naini Tal (Kumaun Hills) U.P., India. Revue Bryologique et Lichénologique, 43: 327–38.
Kashyap, S.R. 1914. Morphological and biological notes on new and little known West-Himalayan Liverworts. I. New Phytologist, 13: 206–26. doi: 10.1111/j.1469-8137.1914.tb05751.x.
Kashyap, S.R. 1929. Liverworts of the Western Himalayas and the Panjab Plain. Part 1. Lahore: The University of the Panjab.
Long, D.G., L.L. Forrest, J.C. Villarreal & B.J. Crandall-Stotler. 2016a. Taxonomic changes in Marchantiaceae, Corsiniaceae and Cleveaceae (Marchantiidae, Marchantiophyta). Phytotaxa, 252: 77–80.
Long, D.G., L.L. Forrest, J.C. Villarreal & B.J. Crandall-Stotler. 2016b. The genus Aitchisoniella Kashyap (Marchantiopsida, Cleveaceae) new to China, and its taxonomic placement. Journal of Bryology.
Pant, G., S.D. Tewari & S. Joshi. 1992. An assessment of vanishing rare bryophytes in Kumaun Himalaya – thalloid liverworts. Bryological Times, 68/69: 8–10.
Rubasinghe, S.C.K., D.G. Long, R. Milne & L.L. Forrest. 2011. Realignment of the genera of Cleveaceae (Marchantiopsida, Marchantiidae). The Bryologist 114: 116-127. http://dx.doi.org/10.1639/0007-2745-114.1.116.
Steinkamp, M.P. & W.J. Doyle. 1981. Spore wall ultrastucture in the liverwort Athalamia hyalina. American Journal of Botany 68: 395-401.
Villarreal, J.C., B.J. Crandall-Stotler, M.L. Hart, D.G. Long & L.L. Forrest. 2015. Divergence times and the evolution of morphological complexity in an early land plant lineage (Marchantiopsida) with a slow molecular rate. New Phytologist. 209: 1734–46, doi: 10.1111/nph.13716.


Praveen Kumar Verma
Excellent work