Quality and quantity of DNA recovered from stored plant material is becoming more important as DNA sequencing technologies change from the relatively simple Sanger sequencing, to next generation sequencing (NGS) technologies such as whole genome sequencing. RNAlater, a proprietary RNA stabilisation solution, first developed and marketed by Ambion is used to recover RNA from collected plant samples as otherwise it degrades very rapidly and cannot be recovered from dried plant collections. DNA can also be extracted from material collected into RNAlater and has been found to be of higher quality than from Herbarium or silica-dried material of a similar age. It was decided to do some comparisons of samples stored in RNAlater with silica dried samples and after reading internet based discussions around solutions that can be used to stabilise RNA, a cheaper homemade buffer solution was also used in the study. The samples in the 2 different solutions were stored for varying periods of time and also at different temperatures, namely room temperature at c. 20°C, at 4°C and -20°C.
The Ambion RNAlater used is product code AM7021, originally purchased from Ambion.
The homemade buffer solution (275ml) is made up as follows:
- EDTA (Ethylenediamine tetracetic acid) 0.5M, pH 8.0 8ml 15mM
- Sodium citrate 1M 5ml 19mM
- Ammonium sulphate 140g 4M
- Purified H2O, autoclaved 187ml
- Sulphuric acid 1M c. 2ml
1.5ml microcentrifuge tubes were filled with 1.3ml of either the proprietary RNAlater or the homemade buffer solution.
Using freshly collected leaves of Alpinia zerumbet (accession 19831003A) from the research glasshouses at RBGE, 10mm discs of material were cut using a core-borer.
The discs were halved (to allow the solution to thoroughly penetrate the tissue) and then immersed into the tubes, 1 disc per tube.
The tubes were kept at room temperature for the first 48 hours and then some stored at 4°C and some at -20°C.
To compare with the quality and quantity of the DNA preserved in the two solutions, discs of the Alpinia were at the same time placed in teabags (5 discs per bag) and then in sealed plastic boxes with silica gel. The boxes were kept at c. 15°C. After 1 week, half of the silica-dried discs were transferred to a humidity controlled cabinet to test the cabinet’s suitability for long-term storage of material.
DNA was extracted from the collected material at intervals; 1 week after collection
(1st row of image below); then 1 month (2nd row); 6 months (3rd row); 1 year (4th row).
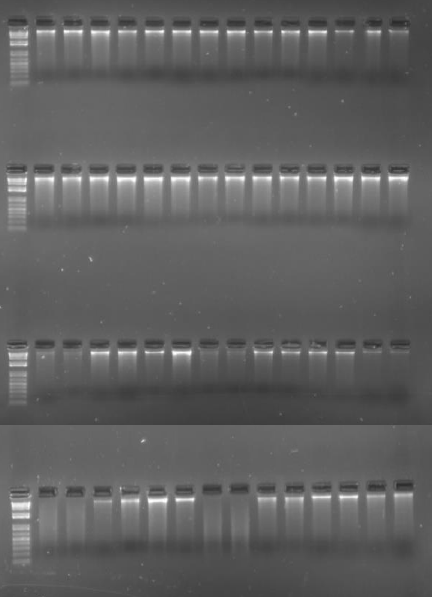
This is an image of the extracted DNA, run out on an electrophoresis gel. The solid white bands indicate high concentrations of large molecular weight (i.e. non-fragmented, good quality) DNA. The smears indicate degraded, lower molecular weight DNA.
| Gel Lane in each row | Preservation Method |
| 1 | DNA size Ladder |
| 2 | Ambion RNAlater, room temp |
| 3 | Ambion RNAlater, room temp |
| 4 | Ambion RNAlater, 4°C |
| 5 | Ambion RNAlater, 4°C |
| 6 | Ambion RNAlater, -20°C |
| 7 | Ambion RNAlater, -20°C |
| 8 | Homemade Buffer, room temp |
| 9 | Homemade Buffer, room temp |
| 10 | Homemade Buffer, 4°C |
| 11 | Homemade Buffer, 4°C |
| 12 | Homemade Buffer, -20°C |
| 13 | Homemade Buffer, -20°C |
| 14 | Silica dried, still in silica |
| 15 | Silica dried, then into cabinet |
While bands of DNA on an electrophoresis gel are a fairly rudimentary indication of quality and quantity, it is clear that storage at room temperature is not the optimum for preserving DNA. It is much better at 4°C and even better at -20°C. Storage of material in both of the solutions compares very favourably with silica-dried material.

The image above shows the leaves after being stored for 1 year, ground up with sand and the extraction buffer. The order of treatment of the samples is the same of the table above, showing brightest green and so best storage, in the tubes that were stored in both solutions at -20°C (6, 7, 12 & 13) and also the silica dried material (14 & 15)
Further tests will include comparisons with another homemade buffer solution – High Salt CTAB (Cetyltrimethylammonium bromide), inclusion of other plant families that are of research interest at RBGE and testing the amount of material that can be stored in each tube without affecting the quality and quantity of DNA.

